
Chemistry
Learning physics
and chemistry
easily and freely - Science for elementary school, middle school and
high school
Free online chemistry lesson for elementary school, middle school and high school.
Acidic and basic solutions
Chemical reaction between iron and hydrochloric acid
The purpose is to study the chemical reaction between iron and hydrochloric acid.
We use:
- Iron powder (Iron powder is more reactive than compact iron )
- A hydrochloric acid solution.
- A test tube which allows a better observation.
Reaction between iron and
hydrochloric acid:
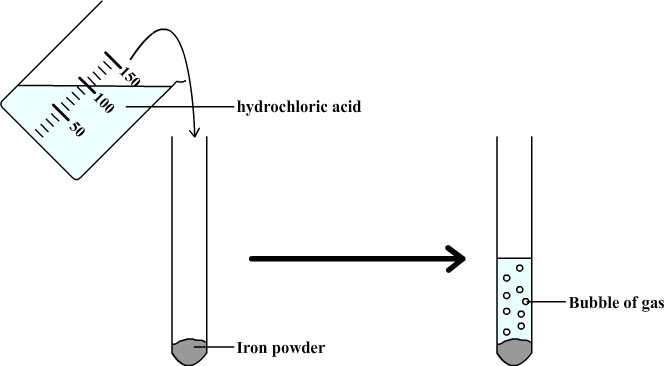

We observe the formation of bubbles which indicate that a gas forms
To understand this chemical reaction we must identify the gaz, and analyze the composition of the aqueous solution in order to check a possible change.
Analysis of the gas:
The test for hydrogen gas is positive. This reaction therefore produces dihydrogen.
Analysis of the resulting solution:
- A measurement of pH shows that the latter increased: the solution is less acidic, hydrogen ions therefore have been consumed.
- The addition of silver nitrate leads to the formation of a white precipitate which indicates that the chloride ions are still in solution.
- The test for iron (II) ions is positive: Iron (II) ions (Fe 2+) have formed.
- The reactants are substances initially gathered: iron and hydrochloric acid.
- Products are substances that have formed: dihydrogen and iron (II) ions that form with chloride ions a solution of Iron(II) chloride
Simple word equation:
| iron + hydrochloric acid | → | dihydrogen + Iron(II) chloride |

©2021 Physics and chemistry


