
Chemistry
Learning physics
and chemistry
easily and freely - Science for elementary school, middle school and
high school
Free online chemistry lesson for elementary school, middle school and high school.
Sates of matter
Molecules in different states of matter
1) Different states of matter
Matter can exist in three different states: solid, liquid or gaseous.
It may also change of state:
From solid to liquid state (fusion or melting)
From liquid to gaseous state (vaporization)
From the gaseous to the liquid state (liquefaction or condensation)
From liquid to solid state (solidification or freezing)
From solid to gaseous state (sublimation)
From the gaseous to the solid state (deposition)
Although matter has different properties depending on its state:
Regardless of the state of a pure substance its molecules are identical.
Example: there is the same molecules of water in ice, in liquid water and water vapor.
2) Molecules in a solid
Schematic representation of molecules in a solid
(Molecules are symbolized by triangles)
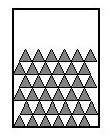
In the solid state, molecules are very close to each other (almost stacked) and the space they occupy is small: they are said to have a compact arrangement.
Each molecule has a fixed position: it is an orderly arrangement.
The solid state is compact and ordered
3) Molecules in a liquid
Schematic representation of
molecules in a liquid
(Molecules are symbolized by triangles)

(Molecules are symbolized by triangles)

L'état liquide est compact et désordonné.
Molecules are still very close to each other and form a compact arrangement. Nevertheless, the molecules are not stationary, they can move by sliding over each other and are slightly agitated: they are organized in a disorderly manner.
The liquid state is compact and disordered.
4) Molécules in a gas
Schematic representation of
molecules in a gas
(Molecules are symbolized by triangles)

(Molecules are symbolized by triangles)

The molecules are relatively far from each other and form a dispersed arrangement.
They are highly agitated and move very quickly in a disorderly manner.
The gaseous state is dispersed and disordered.

©2021 Physics and chemistry


