Chemistry and physics lessons
Chemistry
Free online chemistry lesson for elementary school, middle school and high school.
| _____________________________________________________ _____________________________________________________ |
WaterWater in human body |
|
The essential
about water in human body
Percentage
of water in human bodyLike all living tissues, our body contains water in various amounts: - 75% for a baby - 70% for one child - 60% for an adult 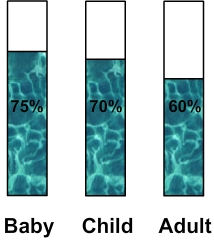 ( diagram: percentage of water in human body ) How much water in human body ? Previous percentages can be used to calculate the amount of water in a human body. For instance: - A baby whose mass is 3 kg: 3 x 75 : 100 = 2,25 kg This baby has 2,25 kg of water in its body ( it corresponds to 2,25 L ) - A child whose mass is 40 kg: 40 x 70 : 100 = 28 kg This child has 28 kg of water in its body ( it corresponds to 28 L ) - An adult whose mass is 70 kg: 70 x 60 : 100 = 42 kg This adult has 42 kg of water in its body ( it corresponds to 42 L ) ______________________________________ ______________________________________ How human body loses water ? The water in our body is eliminated in three ways: - By the urine (approximately 1 L per day) - By sweating (about 0.5 L per day) - By breathing which rejects water vapor (about 0.5 L per day) Water balance in the body To maintain a stable percentage of water in the body and avoid dehydration, lost water must be replaced: Therefore in total 2L of water must be consumed per day: 1.5 from drinks and the balance being provided by food. ______________________________________ ______________________________________ Learn more about water in human
body
The water in you: more detail about percentage of water in various parts of human body and factors that may affect it. The healt benefit if water: how water may affect healt in various ways Drinking too much can kill: article from scientific american that explain water should not be consummed with excess. Water losses of the human body and how to compensate for them: Various factors that may cause losses of water and how to balance them |
|
____________________________________