
Chemistry
Learning physics
and chemistry
easily and freely - Science for elementary school, middle school and
high school
Free online chemistry lesson for elementary school, middle school and high school.
Ions
Tests for ions
The presence of certain ions in an aqueous solution can be detected by making chemical tests.
1) Tests for ions with a precipitation reaction
The presence of certain ions in an aqueous solution can be detected by making chemical tests.
A test for ions is performed in several steps:
- A small part of the tested solution is taken and placed in a test tube.
- A few drops of a chemical called "reactive" are added in this test tube. The reactive is supposed to react with tested ions.
- The result is observed: if the searched ion is present in the solution then a chemical transformation takes place and produces a solid compound which looks a bit like gelatin and that is called "precipitate". Its color must be checked and compared to the expected color.
Example:test for copper ions.
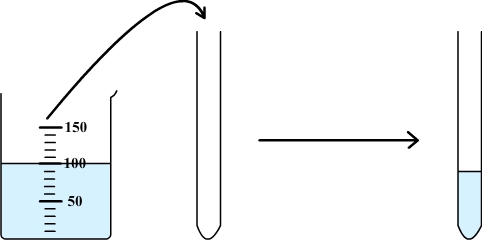
Step
1: some
solution is poured in a test tube

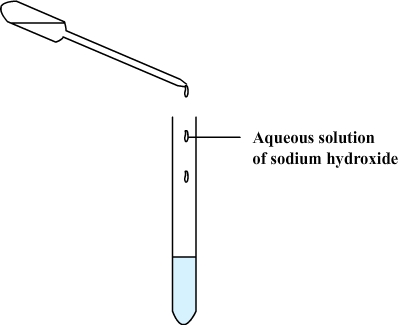
Step
2: A few drops of an
aqueous solution of sodium hydroxide (which
acts as a reactive) are added

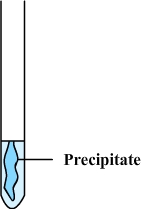
Step
3: A
blue precipitate forms which confirms the presence of copper ions

To realize a test, we must therefore know the reagent to be used and the color of the precipitate that should form.
2) Some tests to know
| Ion | Iron (II) ion Fe2+ | Iron (III) ion Fe3+ | Copper ion Cu2+ | Chloride ion Cl- |
| Reactive | Sodium hydroxide | Sodium hydroxide | Sodium hydroxide | Silver nitrate |
| Color of the precipitate | Green | Red | Blue | White (Blackens in the light) |

©2021 Physics and chemistry


