
Chemistry
Learning physics
and chemistry
easily and freely - Science for elementary school, middle school and
high school
Free online chemistry lesson for elementary school, middle school and high school.
Ions
Formation of ions
1) Formation of monatomic ions
Atoms consist of electrons moving around a nucleus. When some phenomena occur (chemical transformations, mechanical friction, exposure to radiations) the atom may lose one or more electrons.
An electron loss also corresponds to a loss of negative charges that leads to the formation of an electrically charged compound: it is an ion. The electrons lost by one atom can not subsist alone for very long time and are quickly captured by another atom that gains negative charges and also loses its electrical neutrality: this atom is therefore also transformed in ion.
An ion may therefore be formed from an atom that gains or loses one or more electrons.
Comments:
- Such ions that are formed from a single atom are called monatomic ions.
- The number of electrons around a nucleus may change but the nucleus is never changed and it keeps its initial number of positive charges.
2) Formation of a cation
A cation is a positive ion that is formed from an atom that loses some of its electrons.
Indeed, after a loss of electrons, the negative charges become less numerous than the positive charges.
Example: formation of the aluminum ion
The aluminum atom is composed of:
- 13 electrons which carry a total of 13 negative charges.
- 13 positive charges in its nucleus.
This atom can lose 3 electrons to become an aluminum ion which is then made of:
- 13 - 3 = 10 electrons which carry a total of 10 negative charges.
- 13 positive charges in its nucleus (the nucleus is not affected by the loss of electrons)
If we compare the positive and negative charges it can be seen that the aluminum ion has three positive charges in excess (13 positive charges and 10 negative charges).
This excess charge is denoted by a superscript (top right) in the formula of the aluminum ion : Al3+ .
The
aluminum
atom becomes an ion
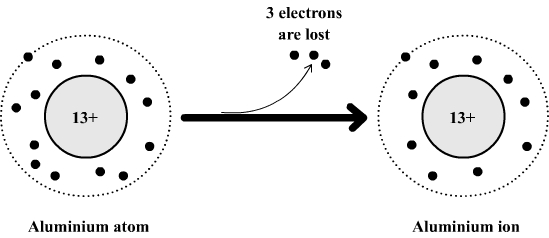

Comment: The formula of an ion is always formed in a similar manner. We use the symbol of the atom from which the ion is formed and then we add in superscript the numbers and the sign of excess charges obtained by comparing the number of positive charges and the number of negative charges.
3) Formation of an anion
An anion is a negative ion that is formed from an atom that gains electrons.
Indeed, after a gain of electrons, the negative charges become more numerous than positive charges.
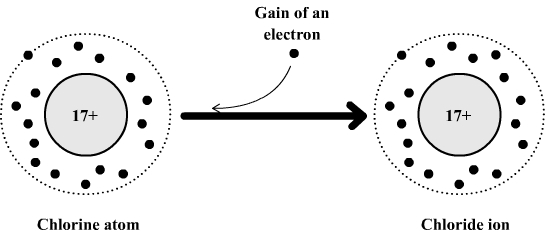
Example: formation of the chloride ion
The chlorine atom is composed of:
- 17 electrons which carry a total of 17 negative charges.
- 17 positive charges in its nucleus.
This atom can gain an electron to become a chloride ion which is then made of:
- 17 + 1 = 18 electrons which carry a total of 18 negative charges.
- 17 positive charges in its nucleus.
If we compare the positive and negative charges we see that the chloride ion has a negative excess charge (17 positive charges and 18 negative charges ).
The formula of chloride ion is: Cl- .
The
chlorine atom become an ion

4) Some ion formulas
The formulas of some common ions
| Sodium ion | Copper ion | Chloride ion | Iron (II) ion | Iron (III) ion |
| Na+ | Cu2+ | Cl- | Fe2+ | Fe3+ |
5) Polyatomic ions
Ions can also be formed from molecules whose atoms lose or gain one or more electrons. These ions are then called polyatomic ions.
Examples of polyatomic ions:
- The sulfate ion whose formula is SO42-
- The carbonate whose formula is CO32-

©2021 Physics and chemistry


