
Chemistry
Learning physics
and chemistry
easily and freely - Science for elementary school, middle school and
high school
Free online chemistry lesson for elementary school, middle school and high school.
Mixtures and solutions
Decantation
1) What is decantation ?
It simply consists in waiting that, in a heterogeneous mixture, constituents separate one each other spontaneously.
2) Example: decantation of a mixture of water and soil
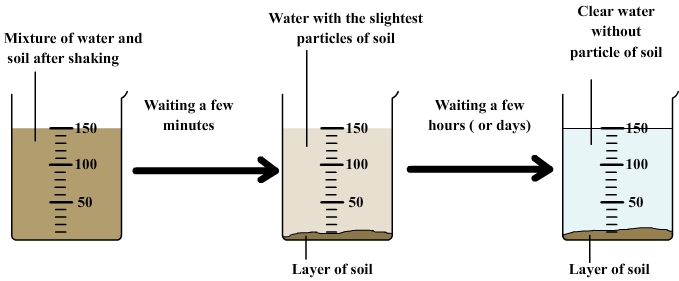
After shaking the mixture, the soil particles are dispersed in water.
We then observe:
- A layer of soil forms slowly to the bottom: it consists of particles that fall as a result of their weight.
- The liquid gradually brightens. Less dense particles fall slower.
- After a sufficiently long time, the liquid becomes clear since all particles have fallen to the bottom of the beaker.
3) Advantages and drawbacks of decantation
Decantation is a simple separation to implement since it requires no special equipment but obtaining a complete separation and a totally clear liquid can takes a long time (sometimes several days) if the particles mixed to water are thin and of low density.
Comments:
- The separation of water and oil after shaking also corresponds to a decantation ( the only difference being that the oil rises to the surface ).
- Decantation also occurs in nature when water is mixed with soil or sand.

©2021 Physics and chemistry


